Reference: 1. Arruda A, Godden S, Rapnicki P, et al. Randomized noninferiority clinical trial evaluating 3 commercial dry cow mastitis preparations: I. Quarter-level outcomes. J Dairy Sci. 2013;96(7):4419-4435.
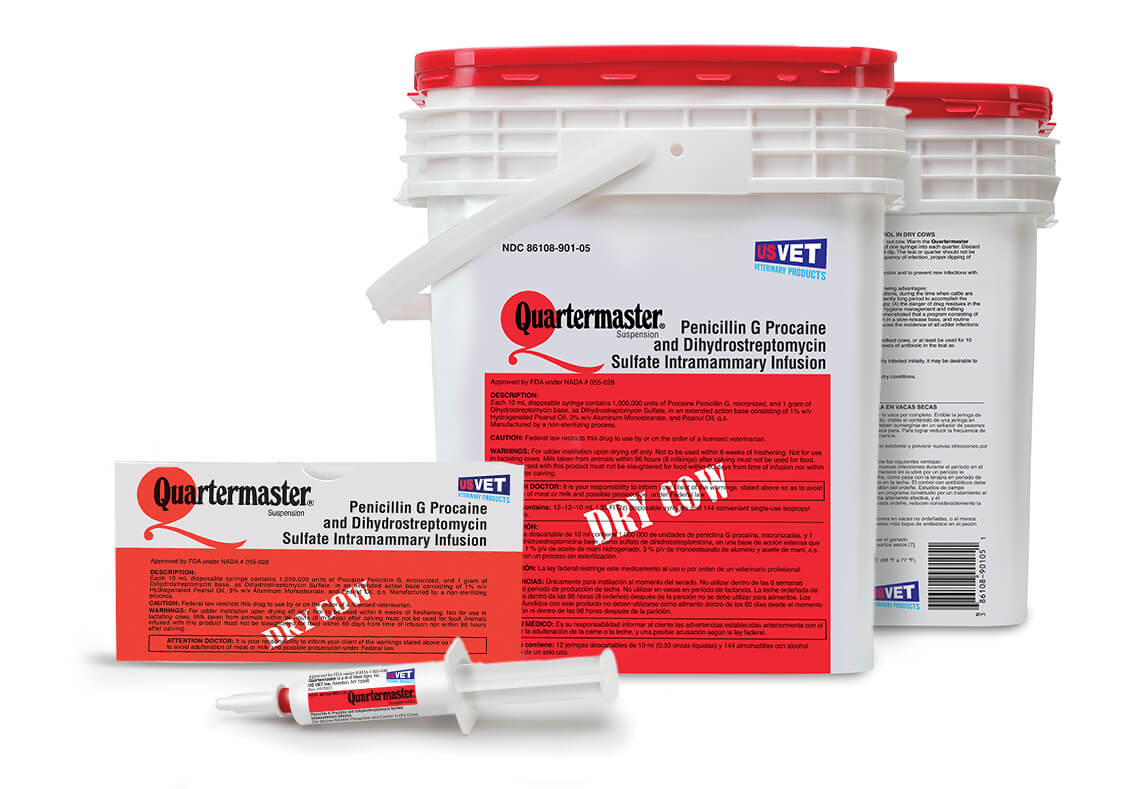
Quartermaster® Suspension Safety Information
Indications
Intramammary use to reduce the frequency of existing infection and to prevent new infections with Staphylococcus aureus in dry cows.
Limitations
Not to be used within 6 weeks of freshening. Not for use in lactating cows. Milk taken from animals within 96 hours (8 milkings) after calving must not be used for food. Animals infused with this drug must not be slaughtered for food within 60 days from the time of infusion nor within 96 hours after calving. Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Ingredients
Each 10 milliliters of suspension contains 1,000,000 units of Penicillin G procaine and 1 gram of dihydrostreptomycin base, as dihydrostreptomycin sulfate.